SiHCl3 (gas) + H2 (gas) ——> Si (solid) + 3HCl (gas)
IV) Chemical purification of silicon
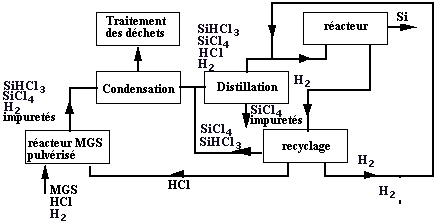
It acts, on the basis of metallurgical silicon to make a chemical purification. One of the methods used, consists in making a distillation of a product, it is liquid at ambient temperature, which contains silicon. A possibility consists in making a silicon halide.
Many processes were developed by the various world producers of silicon based on the trichlorosilane (SiCl4), it is the case for Rhône-Poulenc, Westinghouse, Texas, Saint Gobain, the dichlorosilane (SiH2Cl2)developed by Wacker, or the trichlorosilane (SiHCl3) by Siemens or Union Carbide. Other techniques are based on the tétrafluorosilane (SiF4) or silicon tetraiodure(SiI4).
The chosen example deals with the making of trichlorosilane with pulverization of silicon reacting with hydrogen chloride gas (HCl or hydrochloric acid) according to the reaction:
Si (solid) + 3HCl (gas) ——300°C——> SiHCl3 (gas) + H2 (gas)
For example, as chlorinated metal precipitates are not mixed with trichlorosilane, so the reaction with chlorine allows a first purification. A distillation (like the Alambic one) allows a higher purification then.
This trichlorosilane purified is then reduced to give again silicon in a reactor presented in figure 5. The total chemical reaction is the following one:
SiHCl3 (gas) + H2 (gas) ——> Si (solid) + 3HCl (gas)

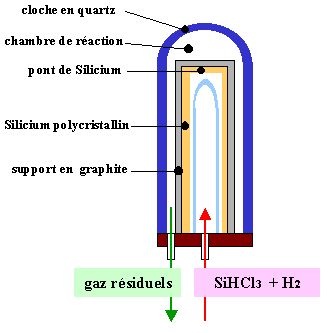
The process is in this case identical to a chemical plating in vapour phase (CVD). From a germ in very long tubes, silicon is gradually deposited. Ingots of polycrystalline structure up to 20 cm in diameter are then obtained. The obtained purity is about the ppm, equivalent concentrations are about1016 cm-3.
The following table shows several examples of the residual concentrations of doping elements after purification. These concentrations are for the most of them low enough to allow using the polycrystalline material for microelectronic applications after growing a crystal. From this polycrystalline silicon the silicon crystal is fabricated. In the following, we will see the several techniques uses from this fabrication.
| Impurity |
|
|
| Al |
|
|
| B |
|
|
| Fe |
|
|
| P |
|
|
| Sb |
|
|
| Au |
|
|